Summary of an integrated experimental and computational approach to study the effects of heavy ion exposures on skin
Jake Pirkkanen,a Claere von Neubeck,b c Marianne, B. Sowa* a
6/25/2014
a Radiation Biology, Pacific Northwest National Laboratory, P.O. Box 999, MS J4-02, Richland, WA 99352, USA
b German Cancer Consortium (DKTK), OncoRay - National Center for Radiation Research in Oncology, Medical Faculty and University Hospital Carl Gustav Carus, Technische Universität Dresden, Fetscherstrasse 74, 01307 Dresden, Germany
c German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, 69120 Heidelberg, Germany
*E-mail: marianne.sowa@pnnl.gov; Fax: +1 509-371-7304; Tel: +1 509-371-6898
High atomic number ion (HZE) exposures occur in space, and are increasingly used in medical exposures (e.g. particle ion therapy). Although the abundance of HZE particles in space is low compared to protons, heavy ions contribute significantly to the effective dose delivered in this environment [14]. Their large mass and high ionization potential generally make them more potent at producing biological damage than comparable X-ray exposures [15, 16]. Ne is one of the predominant atomic nuclei found in cosmic radiation [9]and has also been studied for possible use in clinical therapies [17-22]. Ne has a noble gas core and a first ionization potential of 21.47 eV [9].The Ne ions (300 MeV/u) used in this study have an LET of 35 keV/µm.
An important aspect of the interaction of HZE particles with a tissue is the spatial heterogeneity of the energy/dose deposition pattern as it passes through the sample. The experiments we have undertaken for space like exposures were specifically designed to gain as much information as possible about the details of the radiation deposition patterns on the observed biological responses. A first step in being able to pursue this aim is to obtain a detailed understanding of the tissue construct, its morphology, development of over time and differentiation and proliferation profiles. We have experimentally characterized cellular morphologies in these models by fluorescent and confocal microscopy [23, 24]. We found that the average density of cells in the basal layer is 1.1 x 10-3 cells per µm2. Exposures were therefore designed to deliver either a mean value of one primary ion traversal for every third basal cell (F1 = 3.6 x 10-4 ions per µm2) or one traversal per basal cell (F2 = 1.1 x 10-3 ions per µm2). These fluences emulate exposure levels likely to be experienced by astronauts in the environment of space and deliver a dose of 0.2 and 0.6 cGy respectively.
In addition to the primary ion interactions, energetic secondary electrons (d rays) are produced in large numbers. Using the NASA developed GERM code [25], we estimate the d rays (>1mGy) resulting from the interaction of the Ne ions with the tissue have a range on the order of 60 µm. From our confocal imaging data, we have found the average intercellular spacing in the basal layer to be ~15 µm [23, 24]. Therefore, on average, 4 basal cells will be traversed with a secondary electron for every primary ion traversal. The d rays significantly increase the number of cells that receive some radiation dose and are expected to play a role in the observed responses.
In our study, we exposed 3D skin tissue models to low fluences of high LET Ne ions and measured changes in the thickness of the viable epidermis, the stratum spinosum, and the stratum granulosum. The total numbers of basal cells were also counted. Time points were chosen to characterize short (1, 8, 17 and 26h) and longer term (48, 72, 88 and 120h) effects. Epidermal thickness was observed to increase as a function of time following exposure to low fluence Ne ions in the first 72 hours with F2 having a more prominent effect than F1. The change in epidermal thickness can be due to a change in cell number caused by an increase in proliferation and/or a decrease in the rate of cell clearing via terminal differentiation and sloughing off. We note that changes in cell volume can have an effect on the thickness of the viable epidermis, however, there was no evidence of significant edema. We did not measure proliferation directly in the study reported and Ne ions were not available during subsequent beam time campaigns. However, we were able to gain information on the proliferation rate in the tissue by counting the number of basal cells and looking at immunohistochemical markers of proliferation. The stimulation of proliferation following low dose Ne ion exposures may push the tissue towards a more cancer-like phenotype. This is currently under investigation.
The differentiation profile of the epidermis was determined by measuring regions expressing keratin 10, a cell cytoskeleton protein expressed in the stratum spinosum and granulosum, and filaggrin, a protein that binds to keratin fibers and expressed in the stratum granulosum. The radiation induced time dependent changes in the differentiation profile are complex (See Fig. 3 Ref. [7]). The tissue model continues to develop over time. If one assumes that the primary target of the radiation response is the proliferatively active cells in the basal layer, there will be a delay before these changes would be seen in the stratum spinosum and granulosum. To understand the role of radiation on the dynamics of this process, we have developed an agent based model (ABM) for a skin epithelial tissue model with a finite replicative capacity. ABMs are a set of computational models that can be used to assess the effects of independent events and interactions on a whole system. ABMs are ideal for observing and investigating interactions within diverse populations of cells [28]. ABMs have been used in a cell culture context to understand keratinocyte colony formation [29], and to analyze radiation effects on mammary epithelial cells [30].
Using this model, we examined alternate hypotheses for radiation-induced changes to cell proliferation and differentiation. A base model of the tissue in the absence of radiation was first developed and optimized against experimental observables. This is necessary because the tissue continues to develop over time and has a finite replicative capacity (no stem cells). Several mathematical models have been published to examine development processes in normal human skin [30-32],however this is the first attempt to quantitatively model the cell dynamics in an engineered skin tissue with a finite replicative capacity. The results of our model-based analysis suggest that Ne ion exposures induce a rapid, but transient increase in cell division, which is seen as changes in differentiation status at later times. Our model predictions were independently validated the using histology and qRT-PCR showing its ability to predict a testable hypothesis.
We cannot predict risk accurately following low dose radiation exposure, as too much variability in the epidemiological data will be present. Therefore the future of risk sciences lies in developing integrated experimental and computational models of radiation induced processes that describe the underlying biology with as much mechanistic detail as experimentally and computationally feasible[33]. By pursuing an integrated systems biology research program, such as the one at Pacific Northwest National Laboratory, we are beginning to understand how low dose radiation exposure effects a complex tissue and at various levels of regulation.
Acknowledgements: This work was supported by the National Aeronautics and Space Administration [NNX10AB06G] and the Biological and Environmental Research Program, U.S. Department of Energy [DE-AC06-76RLO]. We would like to acknowledge D. Mullen for assistance with figure preparation and manuscript formatting.
PDF Version
a Radiation Biology, Pacific Northwest National Laboratory, P.O. Box 999, MS J4-02, Richland, WA 99352, USA
b German Cancer Consortium (DKTK), OncoRay - National Center for Radiation Research in Oncology, Medical Faculty and University Hospital Carl Gustav Carus, Technische Universität Dresden, Fetscherstrasse 74, 01307 Dresden, Germany
c German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, 69120 Heidelberg, Germany
*E-mail: marianne.sowa@pnnl.gov; Fax: +1 509-371-7304; Tel: +1 509-371-6898
We live in a world where ionizing radiation is ubiquitous, and exposure to natural sources, both terrestrial and cosmic, has existed throughout evolutionary history. Recently, questions about the degree to which these low level exposures affect human health have increased, largely due to a greater than 6-fold increase in annual effective dose from medical exposures in the last few decades [1]. These concerns are also relevant to those receiving occupational exposures, such as during commercial airline flight, space flight or for radiation workers. The deterministic human health effects of high dose radiation on the cellular level are reasonably well understood, but predicting risks from low dose radiation exposure (<10 cGy) has its challenges [2]. Our approach to bridge the gap between a molecular level understanding of low dose induced tissue level responses and health risk has been twofold. First, we have defined relevant experimental systems to study cellular, molecular, and tissue level biological changes through both global and targeted studies at various levels of regulation including transcription, metabolism and protein expression [3-5]. The various data types have been integrated using bioinformatics to develop a more complete understanding of the system as a whole [6]. Second, predictive computational models have been developed to describe responses to low dose radiation exposures. This mathematical modeling approach is invaluable as it allows us to test multiple hypotheses against experimental data. In the study reviewed here, we performed an integrated experimental and computational study to understand the effects and human health risks of exposure to low doses of space relevant ionizing radiation in a three-dimensional (3D) skin tissue model [7]. We will discuss our choice of experimental models, specific considerations with regards to the radiation interactions in a tissue, and the use of an agent based model (ABM) to interpret the effects of low dose Neon (Ne) ion (300 MeV/u) exposures in a human skin tissue model. ABMs are computational models that can be used to assess the effects of independent events and interactions on a whole system. They are ideal for modeling cellular interactions and responses in this experimental context, as later detailed. All radiation exposures occurred at the NASA Space Radiation Research Laboratory located at Brookhaven National Laboratory.
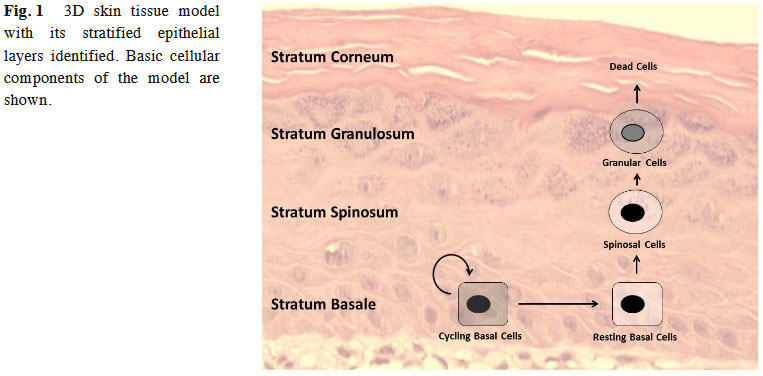
We have chosen skin as an epithelial model because it is a relatively radiation sensitive organ, it will receive a radiation dose in all external exposure scenarios, and can be related to human exposure for validation of the model in the astronaut population. Following low linear energy transfer (LET) radiation exposures, skin is the 4th most responsive organ to cancer incidence [8]and has been proposed as an appropriate model for understanding the cancer development process in epithelial carcinogenesis [9]. In our study, a commercially available, fully differentiated, 3D skin tissue model was utilized. This model, with the inclusion of matrix elements and multiple cell types, better mimics the in vivo environment as compared to 2D in vitro tissue culture systems. It is comprised of a heterogeneous population of keratinocytes in different stages of proliferation and differentiation (epidermis), and fibroblasts embedded in an extracellular matrix (dermis) [ 10]. The basic components of the skin tissue model are identified in
We have chosen skin as an epithelial model because it is a relatively radiation sensitive organ, it will receive a radiation dose in all external exposure scenarios, and can be related to human exposure for validation of the model in the astronaut population. Following low linear energy transfer (LET) radiation exposures, skin is the 4th most responsive organ to cancer incidence [8]and has been proposed as an appropriate model for understanding the cancer development process in epithelial carcinogenesis [9]. In our study, a commercially available, fully differentiated, 3D skin tissue model was utilized. This model, with the inclusion of matrix elements and multiple cell types, better mimics the in vivo environment as compared to 2D in vitro tissue culture systems. It is comprised of a heterogeneous population of keratinocytes in different stages of proliferation and differentiation (epidermis), and fibroblasts embedded in an extracellular matrix (dermis) [ 10]. The basic components of the skin tissue model are identified in
Fig. 1. This model has been extensively utilized to study and measure stress responses in [5 , 11-13]epithelial cells [5 , 11-13].

High atomic number ion (HZE) exposures occur in space, and are increasingly used in medical exposures (e.g. particle ion therapy). Although the abundance of HZE particles in space is low compared to protons, heavy ions contribute significantly to the effective dose delivered in this environment [14]. Their large mass and high ionization potential generally make them more potent at producing biological damage than comparable X-ray exposures [15, 16]. Ne is one of the predominant atomic nuclei found in cosmic radiation [9]and has also been studied for possible use in clinical therapies [17-22]. Ne has a noble gas core and a first ionization potential of 21.47 eV [9].The Ne ions (300 MeV/u) used in this study have an LET of 35 keV/µm.
An important aspect of the interaction of HZE particles with a tissue is the spatial heterogeneity of the energy/dose deposition pattern as it passes through the sample. The experiments we have undertaken for space like exposures were specifically designed to gain as much information as possible about the details of the radiation deposition patterns on the observed biological responses. A first step in being able to pursue this aim is to obtain a detailed understanding of the tissue construct, its morphology, development of over time and differentiation and proliferation profiles. We have experimentally characterized cellular morphologies in these models by fluorescent and confocal microscopy [23, 24]. We found that the average density of cells in the basal layer is 1.1 x 10-3 cells per µm2. Exposures were therefore designed to deliver either a mean value of one primary ion traversal for every third basal cell (F1 = 3.6 x 10-4 ions per µm2) or one traversal per basal cell (F2 = 1.1 x 10-3 ions per µm2). These fluences emulate exposure levels likely to be experienced by astronauts in the environment of space and deliver a dose of 0.2 and 0.6 cGy respectively.
In addition to the primary ion interactions, energetic secondary electrons (d rays) are produced in large numbers. Using the NASA developed GERM code [25], we estimate the d rays (>1mGy) resulting from the interaction of the Ne ions with the tissue have a range on the order of 60 µm. From our confocal imaging data, we have found the average intercellular spacing in the basal layer to be ~15 µm [23, 24]. Therefore, on average, 4 basal cells will be traversed with a secondary electron for every primary ion traversal. The d rays significantly increase the number of cells that receive some radiation dose and are expected to play a role in the observed responses.
In our study, we exposed 3D skin tissue models to low fluences of high LET Ne ions and measured changes in the thickness of the viable epidermis, the stratum spinosum, and the stratum granulosum. The total numbers of basal cells were also counted. Time points were chosen to characterize short (1, 8, 17 and 26h) and longer term (48, 72, 88 and 120h) effects. Epidermal thickness was observed to increase as a function of time following exposure to low fluence Ne ions in the first 72 hours with F2 having a more prominent effect than F1. The change in epidermal thickness can be due to a change in cell number caused by an increase in proliferation and/or a decrease in the rate of cell clearing via terminal differentiation and sloughing off. We note that changes in cell volume can have an effect on the thickness of the viable epidermis, however, there was no evidence of significant edema. We did not measure proliferation directly in the study reported and Ne ions were not available during subsequent beam time campaigns. However, we were able to gain information on the proliferation rate in the tissue by counting the number of basal cells and looking at immunohistochemical markers of proliferation. The stimulation of proliferation following low dose Ne ion exposures may push the tissue towards a more cancer-like phenotype. This is currently under investigation.
The differentiation profile of the epidermis was determined by measuring regions expressing keratin 10, a cell cytoskeleton protein expressed in the stratum spinosum and granulosum, and filaggrin, a protein that binds to keratin fibers and expressed in the stratum granulosum. The radiation induced time dependent changes in the differentiation profile are complex (See Fig. 3 Ref. [7]). The tissue model continues to develop over time. If one assumes that the primary target of the radiation response is the proliferatively active cells in the basal layer, there will be a delay before these changes would be seen in the stratum spinosum and granulosum. To understand the role of radiation on the dynamics of this process, we have developed an agent based model (ABM) for a skin epithelial tissue model with a finite replicative capacity. ABMs are a set of computational models that can be used to assess the effects of independent events and interactions on a whole system. ABMs are ideal for observing and investigating interactions within diverse populations of cells [28]. ABMs have been used in a cell culture context to understand keratinocyte colony formation [29], and to analyze radiation effects on mammary epithelial cells [30].
Using this model, we examined alternate hypotheses for radiation-induced changes to cell proliferation and differentiation. A base model of the tissue in the absence of radiation was first developed and optimized against experimental observables. This is necessary because the tissue continues to develop over time and has a finite replicative capacity (no stem cells). Several mathematical models have been published to examine development processes in normal human skin [30-32],however this is the first attempt to quantitatively model the cell dynamics in an engineered skin tissue with a finite replicative capacity. The results of our model-based analysis suggest that Ne ion exposures induce a rapid, but transient increase in cell division, which is seen as changes in differentiation status at later times. Our model predictions were independently validated the using histology and qRT-PCR showing its ability to predict a testable hypothesis.
We cannot predict risk accurately following low dose radiation exposure, as too much variability in the epidemiological data will be present. Therefore the future of risk sciences lies in developing integrated experimental and computational models of radiation induced processes that describe the underlying biology with as much mechanistic detail as experimentally and computationally feasible[33]. By pursuing an integrated systems biology research program, such as the one at Pacific Northwest National Laboratory, we are beginning to understand how low dose radiation exposure effects a complex tissue and at various levels of regulation.
Acknowledgements: This work was supported by the National Aeronautics and Space Administration [NNX10AB06G] and the Biological and Environmental Research Program, U.S. Department of Energy [DE-AC06-76RLO]. We would like to acknowledge D. Mullen for assistance with figure preparation and manuscript formatting.
REFERENCES
- Mettler, F.A., Jr., et al., Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950 2007. Radiology, 2009. 253(2):p.520-31.
- Authors on behalf of, I., et al., ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs--threshold doses for tissue reactions in a radiation protection context. Ann ICRP, 2012. 41(1-2): p. 1-322.
- Yang, F., et al., Quantitative phosphoproteomics identifies filaggrin and other targets of ionizing radiation in a human skin model. Exp Dermatol, 2012. 21(5): p. 352-7.
- von Neubeck, C., et al., Cell type-dependent gene transcription profile in a three-dimensional human skin tissue model exposed to low doses of ionizing radiation: implications for medical exposures. Environ Mol Mutagen, 2012. 53(4): p. 247-59.
- Varnum, S.M., et al., The effects of low-dose irradiation on inflammatory response proteins in a 3D reconstituted human skin tissue model. Radiat Res, 2012. 178(6): p. 591-9.
- Susan C. Tilton, T.J.W., Bobbie-Jo Webb Robertson, Harish Shankaran, Marianne B. Sowa, David L. Stenoien, William F. Morgan, Katrina M. Waters, Data integration reveals key homeostatic mechanisms following low dose radiation exposure. Toxicology and Applied Pharmacology (Submitted), 2014.
- von Neubeck, C., et al., Integrated experimental and computational approach to understand the effects of heavy ion radiation on skin homeostasis. Integr Biol (Camb), 2013. 5(10): p. 1229-43.
- Thompson, D.E., et al., Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958-1987. Radiat Res, 1994. 137(2 Suppl): p. S17-67.
- Lorz, C., C. Segrelles, and J.M. Paramio, On the origin of epidermal cancers. Curr Mol Med, 2009. 9(3): p. 353-64.
- Boehnke, K., et al., Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur J Cell Biol, 2007. 86(11-12): p. 731-46.
- Curren, R.D., et al., Development of a method for assessing micronucleus induction in a 3D human skin model (EpiDerm). Mutat Res, 2006. 607(2): p. 192-204.
- Schettino, G., et al., Development of a method for assessing non-targeted radiation damage in an artificial 3D human skin model. Int J Radiat Biol, 2010. 86(7): p. 593-601.
- Kandarova, H., et al., In vitro skin irritation testing: Improving the sensitivity of the EpiDerm skin irritation test protocol. Altern Lab Anim, 2009. 37(6): p. 671-89.
- Miller, J., Proton and heavy ion acceleration facilities for space radiation research. Gravit Space Biol Bull, 2003. 16(2): p. 19-28.
- (NCRP), N.C.o.R.P.a.M., Information Needed to Make Radiation Protection Recommendations for Space Missions beyond Low-Earth Orbit: (Report No. 153), 2006: Bethesda, MD
- F. A. Cucinotta, M.H.K., and L. J. Chappell, Space Radiation Cancer Risk Projections and Uncertainties - 2010, 2011, NASA.
- Shigematsu, N., et al., Cell killing and mutation induction by heavy ion beams. Int J Mol Med, 2001. 7(5): p. 509-13.
- Tsuruoka, C., et al., The difference in LET and ion species dependence for induction of initially measured and non-rejoined chromatin breaks in normal human fibroblasts. Radiat Res, 2008. 170(2): p. 163-71.
- Furusawa, Y., et al., Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat Res, 2000. 154(5): p. 485-96.
- Kobayashi, Y., et al., Microbeams of heavy charged particles. Biol Sci Space, 2004. 18(4): p. 235-40.
- Tobias, C.A., et al., Molecular and cellular radiobiology of heavy ions. Int J Radiat Oncol Biol Phys, 1982. 8(12): p. 2109-20.
- Lucke-Huhle, C., et al., Survival and kinetic response of V79-spheroids after exposure to heavy ion beams. Int J Radiat Biol Relat Stud Phys Chem Med, 1980. 37(5): p. 483-92.
- Miller, J.H., et al., Confocal microscopy for modeling electron microbeam irradiation of skin. Radiat Environ Biophys, 2011. 50(3): p. 365-9.
- Miller, J.H., et al., Simulation of electron-beam irradiation of skin tissue model. Radiat Res, 2011. 175(1): p. 113-8.
- Cucinotta, F.A., et al., Nuclear interactions in heavy ion transport and event-based risk models. Radiat Prot Dosimetry, 2011. 143(2-4): p. 384-90.
- Curtis, S.B., et al., A new perspective of carcinogenesis from protracted high-LET radiation arises from the two-stage clonal expansion model. Adv Space Res, 2002. 30(4): p. 937-44.
- Curtis, S.B., et al., The role of promotion in carcinogenesis from protracted high-LET exposure. Phys Med, 2001. 17 Suppl 1: p. 157-60.
- Guillaud, M., C. Clem, and C. Macaulay, An in silico platform for the study of epithelial pre-invasive neoplastic development. Biosystems, 2010. 102(1): p. 22-31.
- Sun, T., et al., An integrated systems biology approach to understanding the rules of keratinocyte colony formation. J R Soc Interface, 2007. 4(17): p. 1077-92.
- Mukhopadhyay, R., et al., Promotion of variant human mammary epithelial cell outgrowth by ionizing radiation: an agent-based model supported by in vitro studies. Breast Cancer Res, 2010. 12(1): p. R11.
- Christley, S., et al., Integrative multicellular biological modeling: a case study of 3D epidermal development using GPU algorithms. BMC Syst Biol, 2010. 4: p. 107.
- Grabe, N. and K. Neuber, A multicellular systems biology model predicts epidermal morphology, kinetics and Ca2+ flow. Bioinformatics, 2005. 21(17): p. 3541-7.
- Edwards, S.W. and R.J. Preston, Systems biology and mode of action based risk assessment. Toxicol Sci, 2008. 106(2): p. 312-8.


